New Treatments For Graves Disease
New treatments for graves disease. Initial results suggest that such application might be helpful in these pursuits although further research is necessary. The medicine called ATX-GD-59 was tested in a phase 1 clinical trial on twelve patients with Graves disease. Teprotumumab is a human-derived monoclonal antibody against IGF1-R effectively blocking the inflammatory responses of IGF1-R.
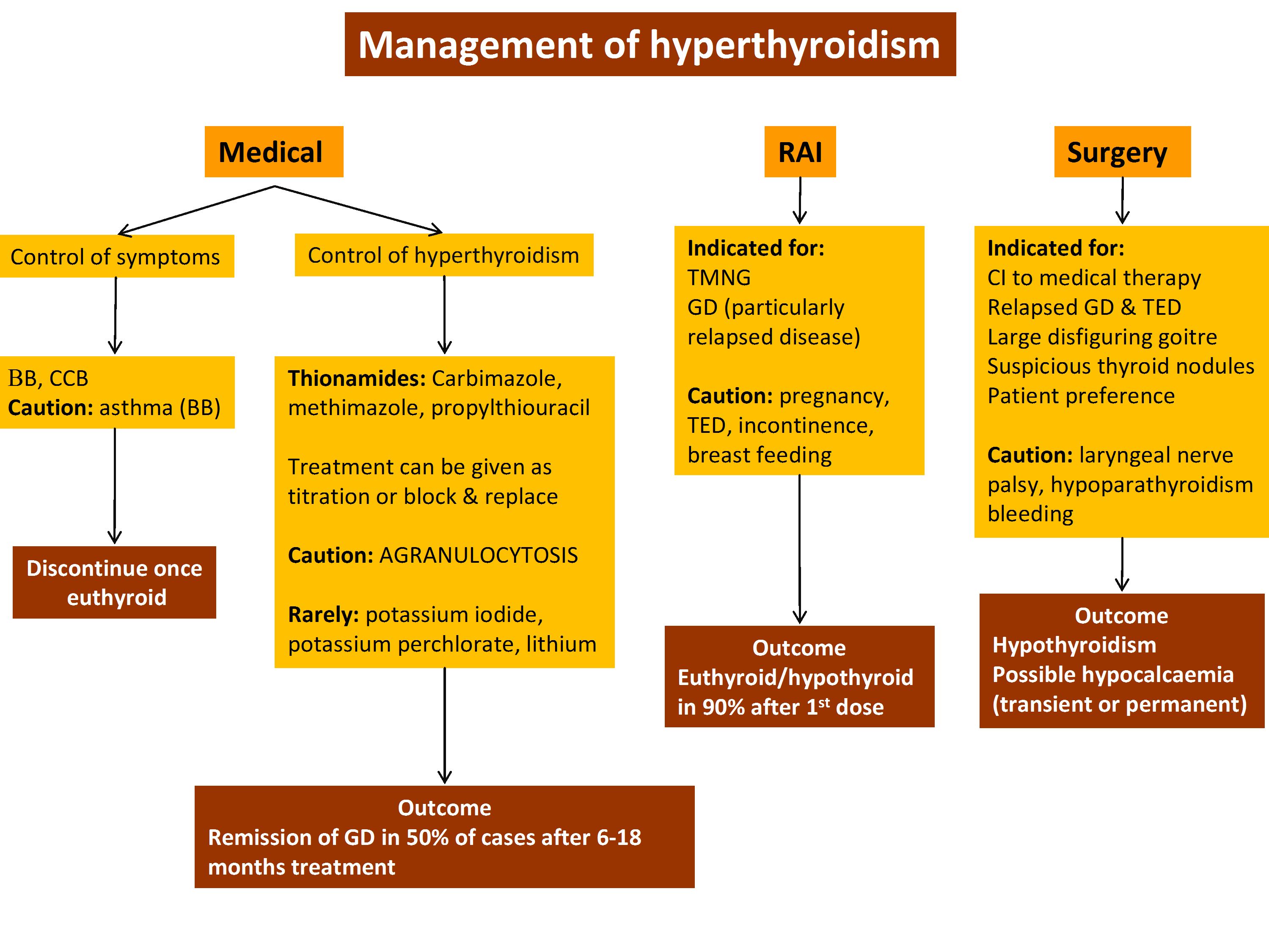
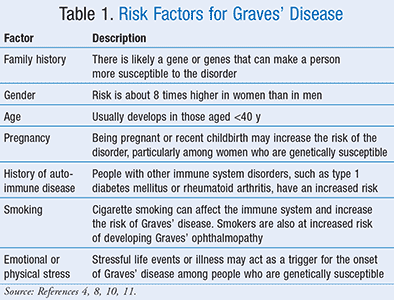
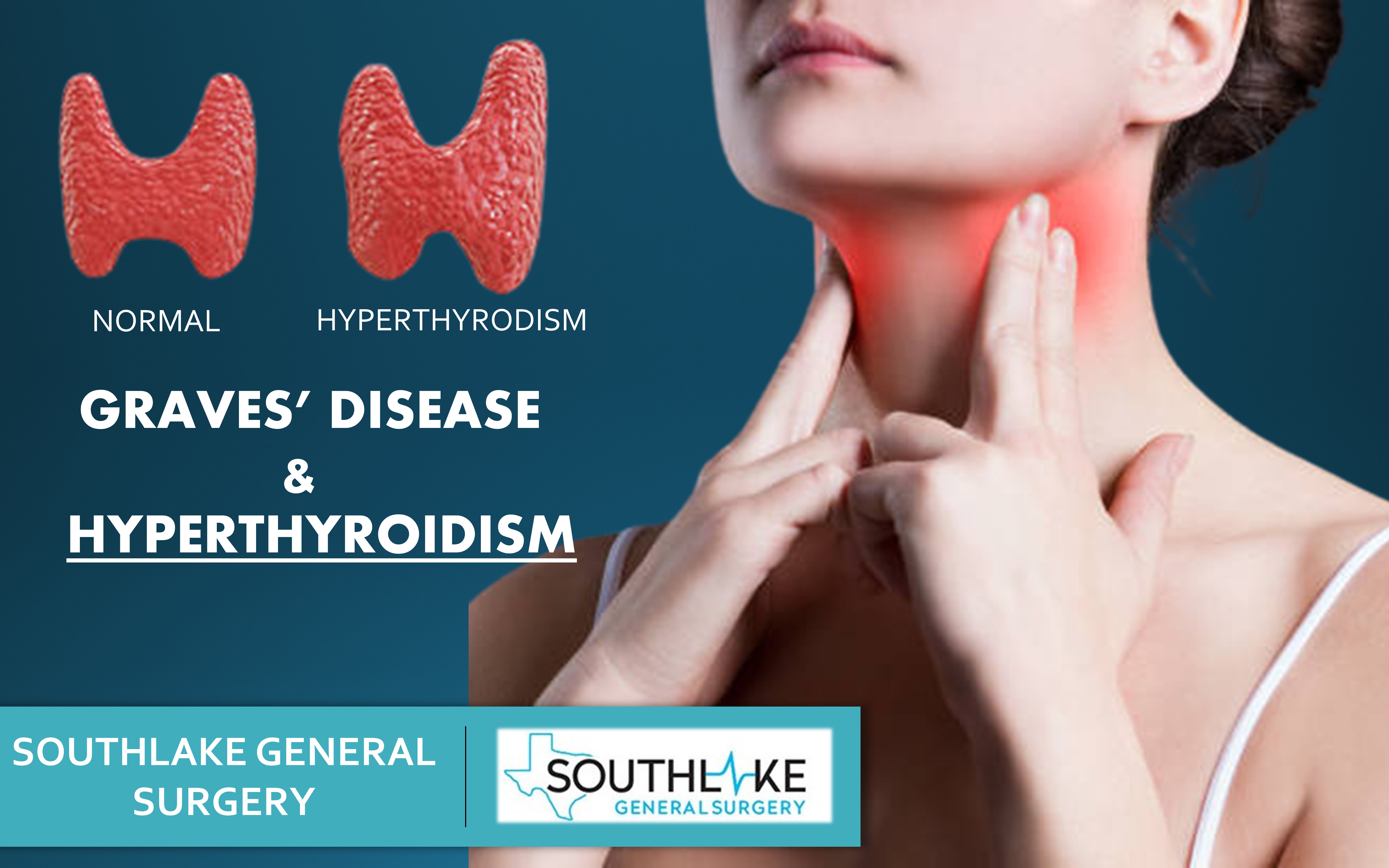
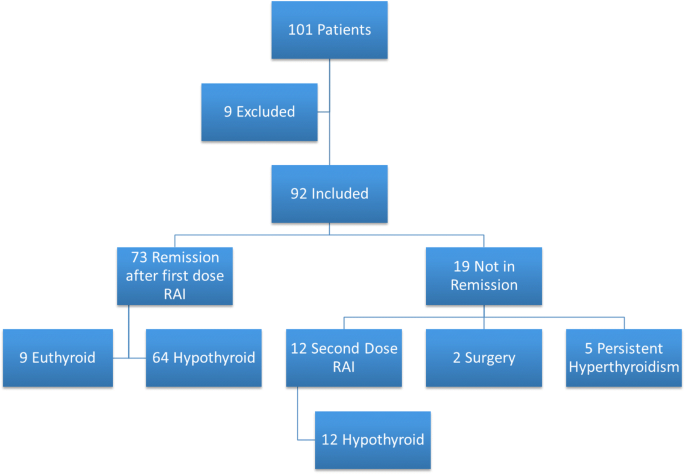
People who are diagnosed with hyperthyroidism in particular Graves disease are treated with antithyroid medication radioactive iodine RAI therapy or surgery but the long-term effects of each choice are not well known. Further if a family member has Graves disease it is more likely for other family members to develop it. Radioiodine represents a cost-effective treatment option for Graves disease.
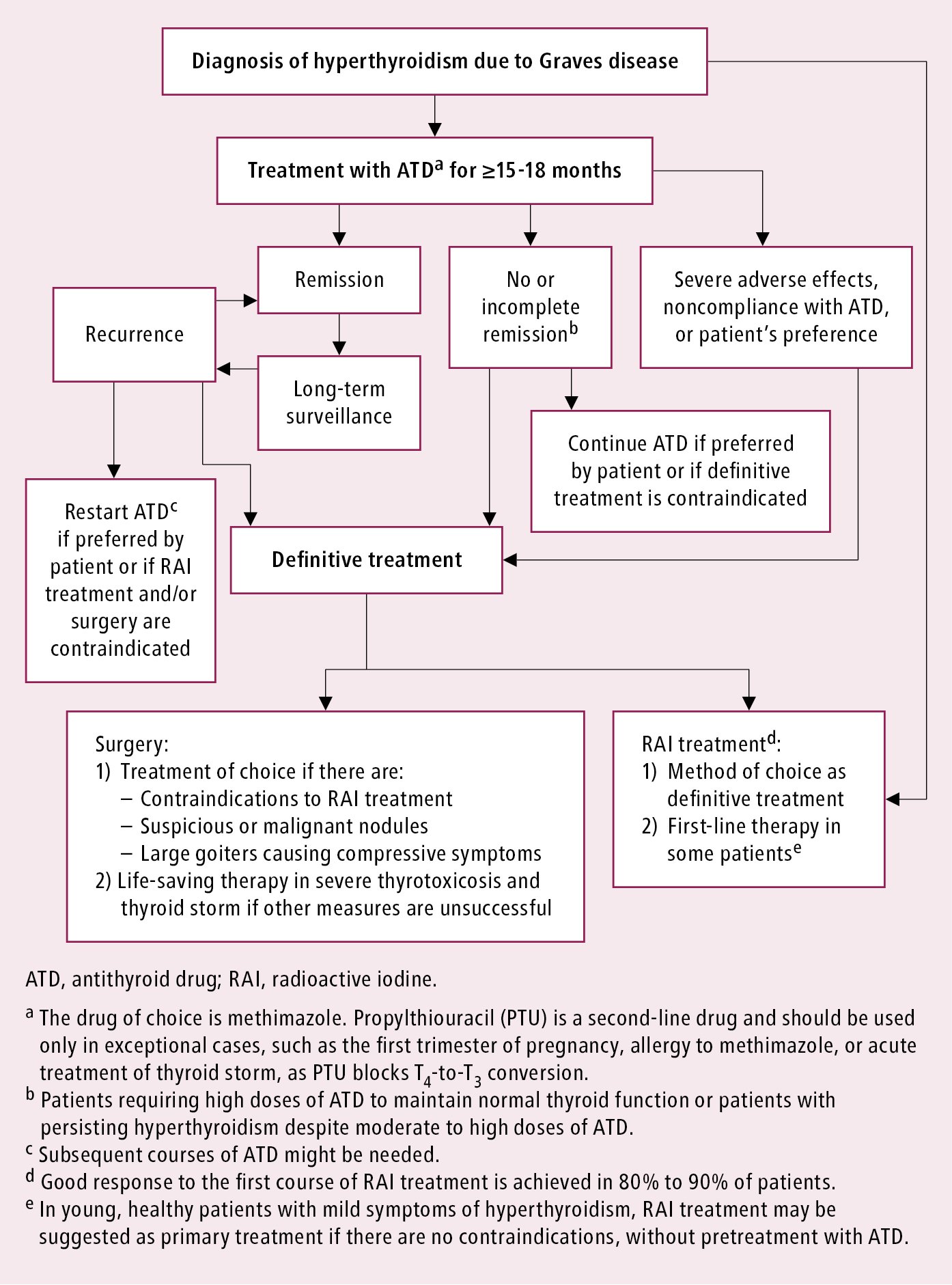
We will continue to progress the development of ATX-GD-59 as a treatment for Graves disease and are currently preparing to initiate a Phase II study in the first quarter of 2019 The investigational product was administered intradermally ID every two weeks over a period of 18 weeks in male and female patients that were not otherwise being treated with anti-thyroid therapy. Basic blood work for Graves disease should include TSH thyroxine T4 triiodothyronine T3 and a complete blood count. In recent years there has been a shift towards antithyroid drugs including long term therapy with these agents given the advantage of avoiding hypothyroidism and the apparent safety of this approach.
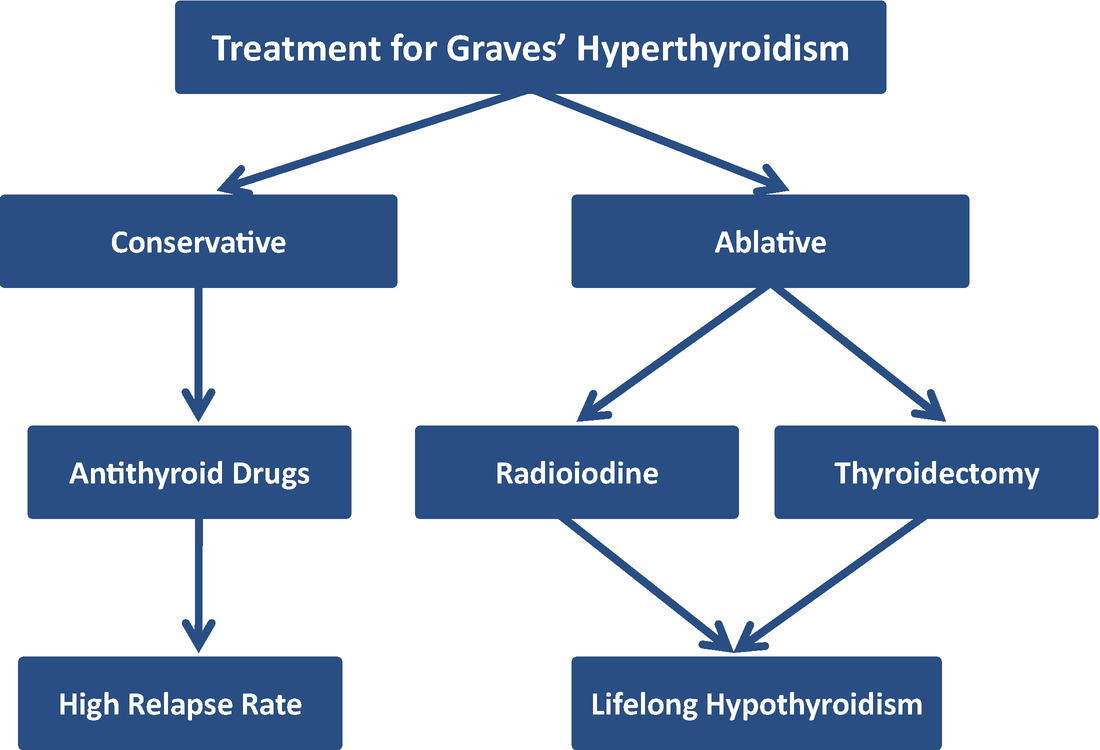
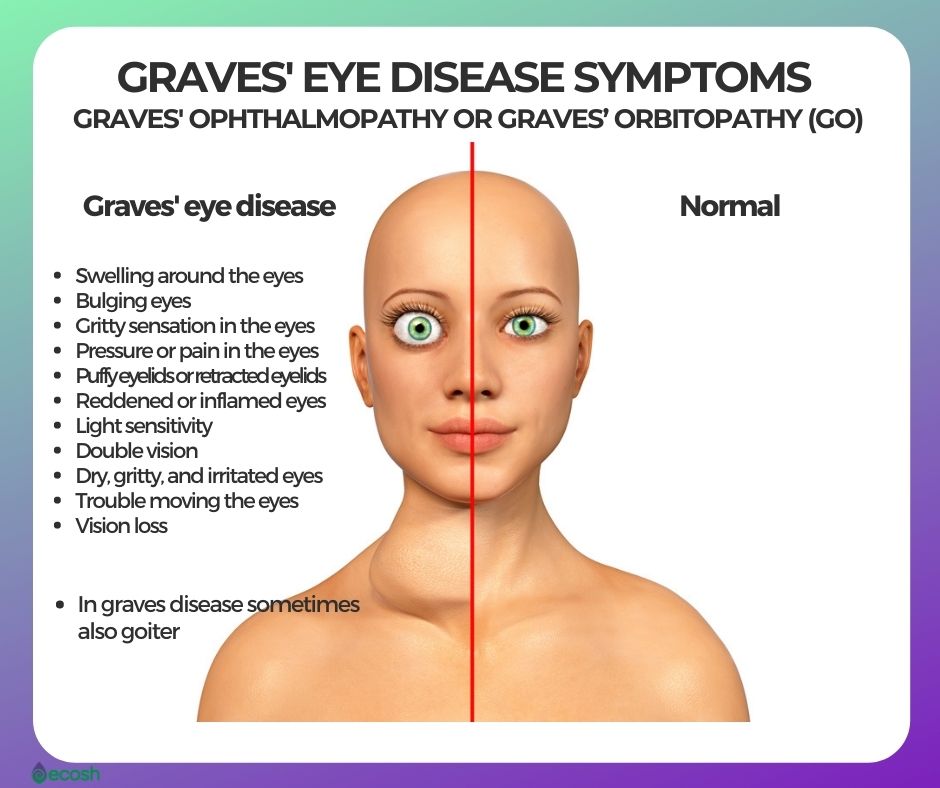
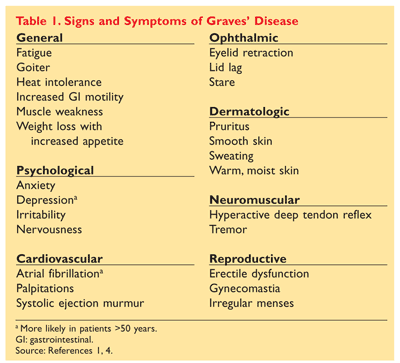
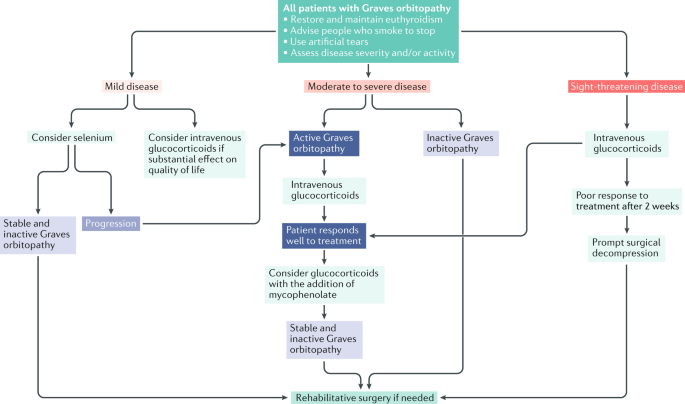
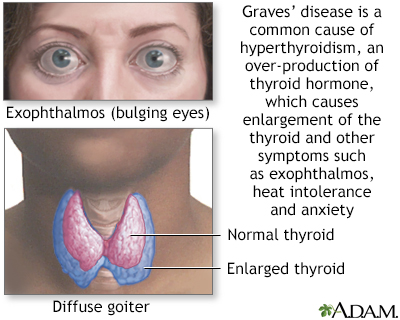
Graves disease is classically managed with one of three treatment options - antithyroid drugs radioactive iodine and thyroidectomy. There has never been a safe and effective treatment for Graves eye disease also known as thyroid eye disease TED for the 1 million Americans with the condition. Graves disease occurs in approximately 1 out of 200 people and most commonly affects individuals between the ages of 3050.
In January of 2020 Teprotumumab was approved by the FDA for the treatment of TED in Graves Disease patients. Dealing with Graves disease. There are also studies that have examined alternative treatments for Graves disease such as using royal jelly cream derived from worker bees which is said to possess anti-inflammatory and immunomodulatory properties.
The new immunotherapy medicine being tested by Apitope would provide a significant development in the treatment of this disease. I am not associated with The Thyroid Trust - just a person living with Graves and excited about that idea that there might be something new coming on line for us. January 22 2020.
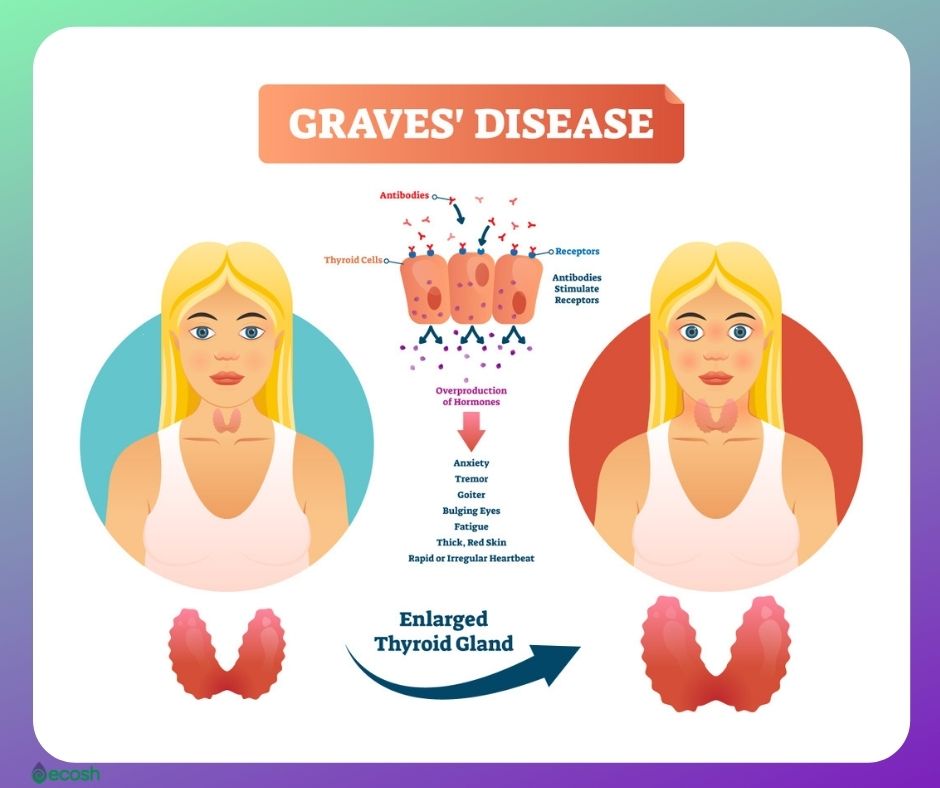
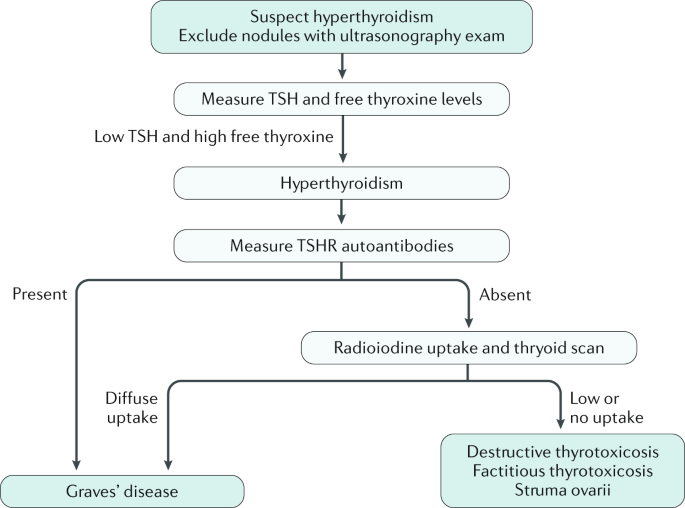
It is 7 times more common in women than in men. TSI thyroid-stimulating immunoglobulins tests for a group of antibodies that cause hyperthyroidism.
In the UK it is traditionally reserved for patients who relapse after initial thionamide therapy.
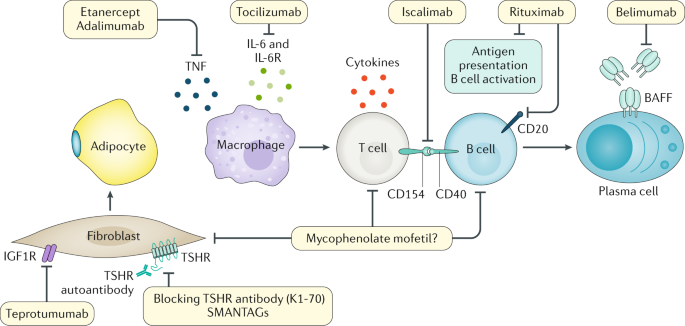
The new immunotherapy medicine being tested by Apitope would provide a significant development in the treatment of this disease. There has never been a safe and effective treatment for Graves eye disease also known as thyroid eye disease TED for the 1 million Americans with the condition. Further if a family member has Graves disease it is more likely for other family members to develop it. It is free to attend or you can make a donation. Graves disease is classically managed with one of three treatment options - antithyroid drugs radioactive iodine and thyroidectomy. New Findings Show Promise for Treatment of Graves Disease and Other Ocular Disorders. Current therapeutic options for the treatment of Graves hyperthyroidism that are discussed below include those directly targeting the B cells or their associated interactors and cytokines or alternatively specific TSHR modulation by the use of small molecule antagonists antagonistic TSHR monoclonal antibodies or tolerogenic TSHR peptides. Aryl hydrocarbon receptor ligands that block myofibroblast formation and collagen production in thyroid eye disease may be the key according to a new report in The American Journal of Pathology. The new immunotherapy medicine being tested by Apitope would provide a significant development in the treatment of this disease.
Dealing with Graves disease. Graves disease occurs in approximately 1 out of 200 people and most commonly affects individuals between the ages of 3050. Current therapeutic options for the treatment of Graves hyperthyroidism that are discussed below include those directly targeting the B cells or their associated interactors and cytokines or alternatively specific TSHR modulation by the use of small molecule antagonists antagonistic TSHR monoclonal antibodies or tolerogenic TSHR peptides. Aryl hydrocarbon receptor ligands that block myofibroblast formation and collagen production in thyroid eye disease may be the key according to a new report in The American Journal of Pathology. In the UK it is traditionally reserved for patients who relapse after initial thionamide therapy. New Therapeutic Horizons for Graves Hyperthyroidism. It is 7 times more common in women than in men.









/3232992_color2-5c40ebe0c9e77c0001cc2a11.png)

:max_bytes(150000):strip_icc()/overview-of-hypothyroidism-4164534_final-b72106e4545d45afad2caff2910427d9-1697410c70574bc29facb17e0024a9d8.png)




















Post a Comment for "New Treatments For Graves Disease"